New skin sensitisation guideline
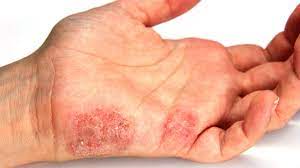
ECHA has published advice on how to use the OECD guideline to REACH registrants on how to reliably combine different sources of non-animal data on skin sensitisation properties of their substances. This is the first guideline outlining how to use in silico tools like the QSAR Toolbox for this purpose. Mike Rasenberg, ECHA’s director for hazard assessment, described it as “an important milestone for advancing the use of alternative methods to assess chemical hazards”.
The guideline contains defined approaches for assessing whether a substance is a skin sensitiser and categorising the sensitisation as strong or moderate, which is required under REACH. If the defined approach results in a conclusion, it can replace the Local Lymph Node Assay, reducing testing on animals.