Quotient integrates drug substance into platform
Submitted by:
Andrew Warmington
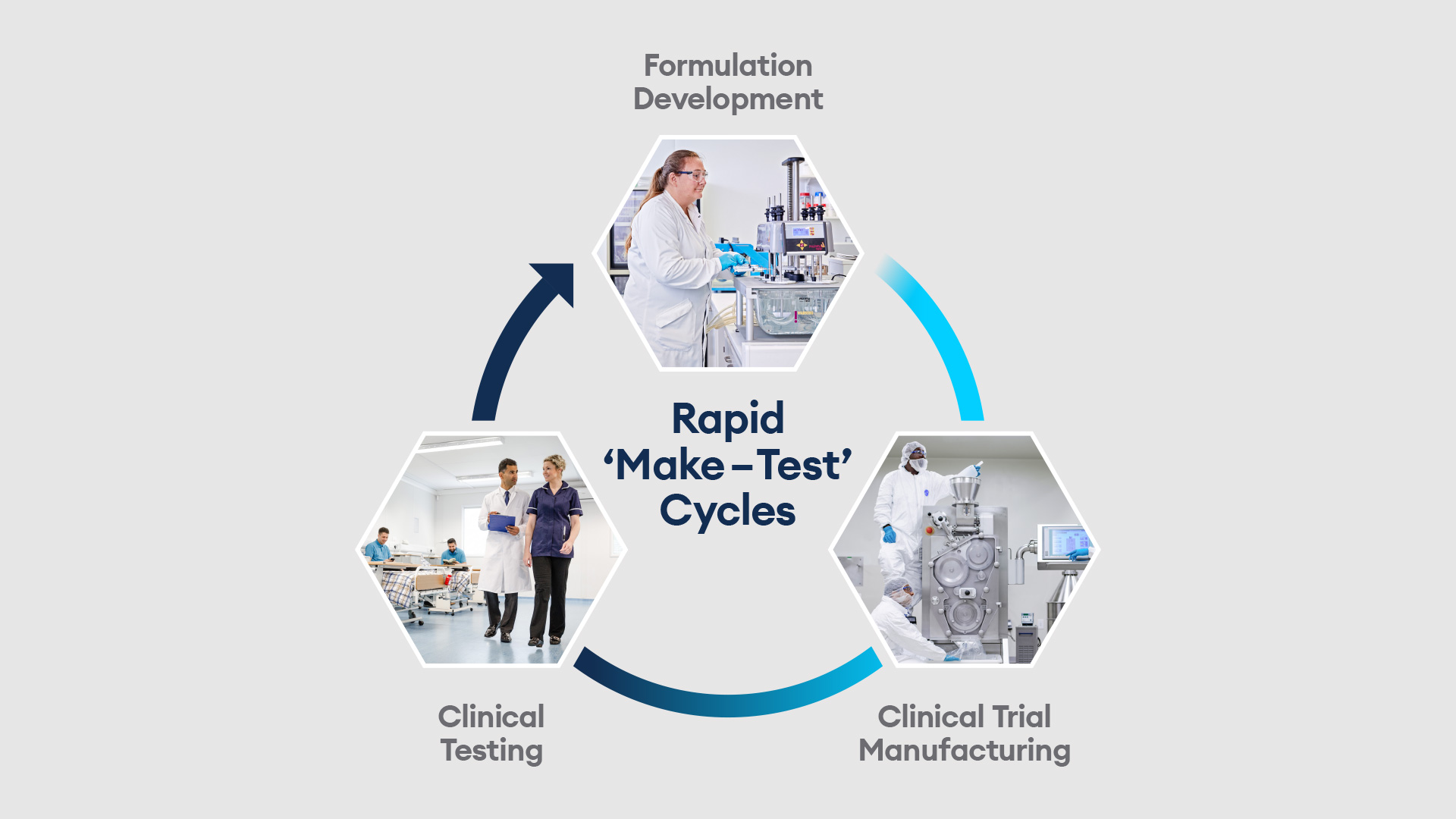
Quotient Sciences, a UK-based firm that describes itself as a “drug development and manufacturing accelerator”, has integrated drug substance into its flagship Translational Pharmaceutics platform, uniting it with drug product and clinical testing activities within one organisation under a single project manager. This follows a year after the acquisition of the former Arcinova site at Alnwick. The company said that Translational Pharmaceutics “provides a more streamlined approach from candidate selection through to commercialisation”, enabling users to adjust formulations and dosing in real time.
Now in its 15th year, Translational Pharmaceutics was developed in consultation with the MHRA and FDA. It uses a rapid ‘make-test’ cycle, in which drug products are manufactured, released and dosed in a clinical study in days rather than months. It has been used on about 500 drug developments programmes to date.
According to a study by the Tufts Center of the Study of Drug Development, the programme has saved $200 million in drug development costs per approved drug. “This approach is proven to shave 12 months off timelines and, by adding drug substance synthesis, the timeline from candidate selection to clinic can be further accelerated by two to four months,” said Quotient CEO Mark Egerton.
Quotient has also recently signed a global licensing agreement, permitting TRx Biosciences to use Translational Pharmaceutics in the formulation development and preparation of clinical trials for Oxilio’s OXL001. This follows an agreement to use the platform in developing OXL001 that was signed in October 2021.
Oxilio is a drug development company focused on repurposing and commercialising existing drugs to address unmet needs in cancer therapies. TRx itself has developed technology for targeted oral drug delivery to specific organs, cells and tissues in cancer treatment, among various other major diseases.
Oxilio also has an ongoing service contract with Quotient to support the formulation development of NXP001, a new form of aprepitant, which it has licensed globally to fibrosis and cancer drug development company Nuformix. The two firms are seeking to identify and evaluate a co-crystal formulation to deliver optimal bioavailability for the treatment of cancer chemotherapy-induced nausea and vomiting.
Quotient has already worked with the co-crystal, and developed a capsule and a powder for oral suspension formulation, evaluating its performance in a relative bioavailability study versus a branded aprepitant. It will now prepare CMC batches and stability data for Oxilio to support a clinical trial application for the new formulation.
